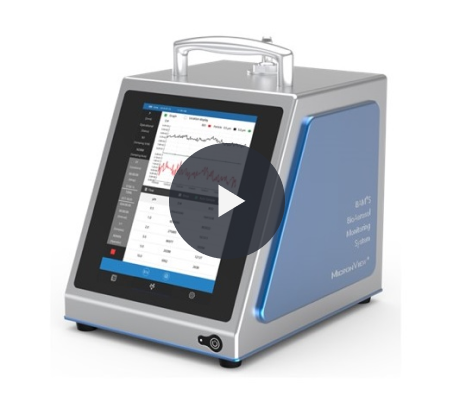
BAMS BioAerosol Monitoring System
The first truly portable continuous, real-time microbial monitor. BAMS is an airborne particle counter used to detect both inert and microbial particles in real-time
MONITORING AIRBORNE MICROBES IN REAL-TIME
BAMS was designed to meet exacting, pharmaceutical manufacturing standards while providing real-time data for immediate action and catastrophic loss avoidance
The BioAerosol Monitoring System (BAMS) is an airborne particle counter used to detect both inert and microbial particles in real-time.
The first truly portable continuous, real-time microbial monitor, that uses Laser-Induced Fluorescence to differentiate biologic from inert particles, with viable particles emitting fluorescence.
Key benefits of BAMS
What makes the Bio-Aerosol Monitoring System an essential part of your Environmental Monitoring programme?
Continuous
Real-time, continuous airborne microbial monitor
Immediate
Certified ISO particle detector
efficient & Effective
First truly portable, user-oriented microbial monitor with no consumables
Root Cause
A uniquely effective diagnostic tool, BAMS can instantaneously help detect excursions and help identify the root cause.
Trends
Given delays and time lapses inherent to compendial testing methods, trend analysis is all but prohibited. BAMS changes that.
Alerts
BAMS provides real-time continuous data to help with the root cause identification of contamination. Delivering alerts in-time to reduce the risk of product loss.
Process & Training
BAMS real-time results are a perfect training aid to drive immediate technique correction and process improvement.
Sterility Test Isolators
BAMS enables enhanced coordination and control of sterility test isolators.
Fill Line Quality
BAMS continuous monitoring helps to ensure the cleanliness of this crucial quality environment.
Learn more about BAMS
Discover more about the innovative Bio-Aerosol Monitoring System
FEATURES OF BIOAEROSOL MONITORING SYSTEM
Wait Time vs Real Time
Current airborne microbial monitoring uses interval, ad-hoc and event-driven sample collections, which require incubation, taking 1-7 days to generate test results, delaying and, at best, inhibiting, contamination root cause identification.
The current monitoring process also requires managing complex collection and manual growth examination schedules for thousands of air samples per month.
Increased Control
BAMS was designed to meet exacting, pharmaceutical manufacturing standards while providing real-time data for immediate action and catastrophic loss avoidance.
It is designed for end-users and is small light and easy to use. Making BAMS the first truly portable continuous, real-time microbial monitor.
Optical Sensor Technology
BAMS principle of operation is the simultaneous measurement of an individual particle’s size and its ultraviolet (UV) induced intrinsic fluorescence signal.
Particle sizing is possible through the widely utilised principle of Mie scattering.
Simultaneously, the instrument detects the presence or absence of the intrinsic fluorescence
of certain metabolites that indicate biologic activity.
NEED SOMETHING ELSE?
Browse our products and services catalogue
FREQUENTLY
Asked Questions
Typical requirements suggest 1,000 litres per air sample in high risk areas, such as: grade A filling lines, grade B clean rooms, operating theatres etc. As the criticality of the area reduces, the sample size can be reduced. The aim is to achieve a representative sample; so where higher counts would be expected, a smaller sample produces a more realistic number of cfu to count.
SAS samplers were originally designed for Contact plates, however, a Petri dish option has been available for a number of years. It is really a personal choice, although this should be decided at time of purchase, as the sampler will be specifically configured for the plate type chosen. There are advantages for each version and we would be happy to discuss your specific needs.
No, do not put your SAS sampler in a steam autoclave. The only part that can be autoclaved is the drilled head. The unit can be wiped with alcohol wipes to decontaminate it. The only other exception is the SAS Pinocchio, parts of which can be autoclaved.
Cherwell Laboratories recommends every 12 months and we will send a reminder for the month it is due. For some situations local procedures demand more frequent recalibrations so Cherwell is happy to offer tailored recalibration date labelling and reminders on request.
The sterility of the packaged medium is assured and all but the outer layer of packaging is also sterile. Thus the risk to the environment to be sampled is greatly reduced. There is an additional benefit that the additional packaging and process extends the room temperature shelf life. This can be sufficient reason for small or irregular users to prefer irradiated.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
The storage condition for the majority of our prepared media is Ambient not exceeding 25ºC, the exception being a couple of very specialist products.
We have never specified storage in a fridge for our general media as this causes excessive condensation and can result in a very wet agar surface. This makes the product impossible to use.
General purpose media have nutrients that support the growth of most non fastidious culturable microorganisms. Selective growth media contain components that will inhibit the growth of some types of microorganisms, while supporting the growth of others.
General purpose media, such as Tryptone Soya Agar, are used to produce total counts. While selective media, such as XLD for Salmonella species, are used to test presence/absence of specific types of microorganism.
CHERWELL IS DIFFERENT
Supplying products is only the start
The best products prepared for you
Our Redipor® media products are batch tested, QC certified and delivered when you need them
Bespoke accessories and services
We provide additional equipment or services to make your environmental monitoring more effective
Best customer service
We build long-standing client relationships on understanding, commitment and trust