Growth Media Types
Understanding the different types of prepared media and how they are used in environmental monitoring, sterility testing and process validation is crucial in selecting the right product
PREPARED MEDIA IS USED IN ENVIRONMENTAL MONITORING AND TESTING APPLICATIONS

Healthcare, pharmaceutical and industrial organisations use reliable, quality assured prepared media every day
Cherwell Laboratories has been manufacturing prepared microbiological media under the Redipor® name since the early 1980s and has built a strongly regarded reputation within the pharmaceutical and healthcare industries for high quality products and services.
Over the years we’ve embedded ourselves as a ‘go to‘ manufacturer that has in-depth industry knowledge, which allows us to appreciate high quality culture media cannot be understated for microbiological purposes in the pharmaceutical and cleanroom industry
Our team have extensive experience and understanding of our product portfolio; the applications they are used for; the appropriate standards and regulatory requirements and quality in microbiology. They are always available to offer practical advice and solutions tailored to meet individual customers’ specific microbiological monitoring and validation needs.
There is a diverse range of prepared culture media available on the market that cover many industries and sectors performing in the microbiology space. Our Redipor® range is predominantly focused on the needs for environmental monitoring (EM), sterility testing and operator and process validations. Our range is too extensive to list entirely and covers both general purpose and selective growth media. Here’s an overview of our most commonly used media types, including their primary uses and applications. Click on the links below to gain a more in-depth insight.
We’ve focused on the main media types supplied for EM work, sterility testing and process validations. However, if you have any questions regarding a media not listed here, then please do contact us, our team of experts are only too happy to help.
Page contents
Tryptone Soya Agar
Also known as Casein Digest Peptone, TSA is the primary medium used for environmental monitoring applications within pharma because it will grow a wide range of bacteria and mould. Referenced in the pharmacopoeia, TSA is a pale straw-coloured media that is particularly good at withstanding the effects of gamma radiation due to a low content of sugars.
The limits within GMP for viable particles within cleanrooms are all based on the use of TSA in Settle plates and active air samples.
Relevant products:
Gamma Irradiated Media
Poured Culture Media Plates
Relevant content:
Incubating Plates - Challenges and Lessons Learnt
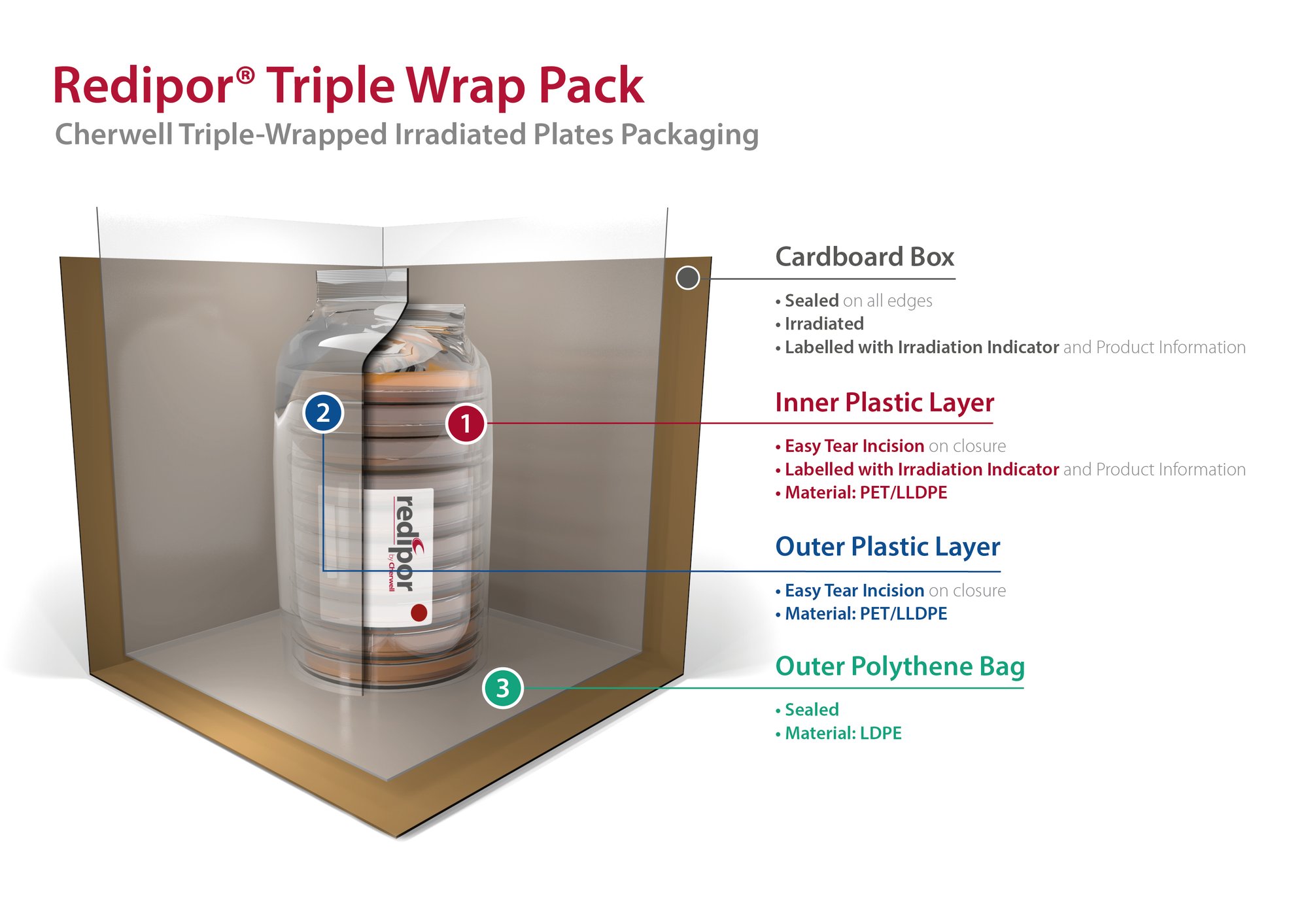
Redipor® Triple Wrap Packaging Illustration
Tryptone Soya Agar plus Neutralisers
A noticeable development within pharma EM over the last 10 years has been the increasing use of media supplemented with disinfectant neutralisers. The common supplements were Lecithin and Tween, but this has been surpassed by a 4 neutraliser mix which also includes L-Histidine and Sodium Thiosulphate.
Neutralisers are typically used in Contact plates for surface monitoring, but increasingly users have also included them in Settle plates to counteract disinfectant residues from finger dabs.
Relevant products:
Gamma Irradiated Media
Poured Culture Media Plates
Relevant content:
Why Use Neutralisers in your Environmental Monitoring Plates?
Researching Disinfectant Neutralisers
Are all Contact plates the same when it comes to EM?

Tryptic Soy Broth/Tryptone Soya Broth
The main medium for sterility testing, TSB is the liquid version of TSA, mentioned above, with the absence of agar to set the medium. A mid to dark brown liquid it is presented in many different containers, from 5ml Injection vial, to Ampoules and Broth Bags, all to allow users to assess the sterility of a product, process or operator.
As with most liquid growth medium, contamination shows as turbidity of the normally clear liquid.
Relevant products:
Injection Vials and DIN Bottles
Universal Operator Broth Transfer Kit
Bottled Media Products
Broth Bags and Ampoules
Relevant content:
Cherwell to Introduce New Redipor® Broth Kit at APDM
Case Study: Great Ormond Street Hospital (GOSH)
Sabouraud Dextrose Agar
SDA is a mould and fungi specific agar that can be used to supplement EM programs, especially in slightly lower grade facilities where the presence of mould and fungi could be more likely. Sabouraud Dextrose agar is very similar in colour to TSA, so users need to be aware of the risks and implications of mixing the two agar types.
The increasing reliance on dual incubation within pharma EM for TSA has significantly reduced the amount of SDA used.
Relevant products:
Gamma Irradiated Media
Poured Culture Media Plates
Relevant content:
Considerations for Environmental Monitoring Plates
Redipor® Contact Plate Grip Lids Demonstration Video
Redipor Triple Wrap Packaging
This diagram shows our Redipor® Triple-wrapped irradiated plates packaging. It comprises of three layers, with two layers of flow wrap and a large sealed polybag.
The illustration demonstrates the sterility assurance with each layer, following exposure to gamma radiation.
It allows the plates to be easily handled without the risk of introducing contaminants, while transporting product through each stage of a facility. Maintaining conditions for the agar and providing consistent, reliable prepared media that delivers confidence in sampling.
This is a representation of our standard triple wrap that can be applied across our range of plated product. We do offer other presentations, please contact us for more information.
Fluid Thioglycollate Medium
Also known as FTM, Thioglycollate is a specific growth medium for anaerobic organisms. Its main application is again within sterility testing but specifically to identify anaerobic organisms within a sample or product.
A key performance parameter for FTM is the presence of resazurin, an indicator of aerobic conditions within the medium. This can typically present itself as a shallow pink band at the top of the medium. This doesn’t affect the performance of the medium, and if need be, can be driven off by gently heating the medium.
Relevant products:
Injection Vials and DIN Bottles
Bottled Media Products
Relevant content:
Cherwell provides CMO with bespoke broth for method validation
Prepared Microbiological Media: Pros and cons

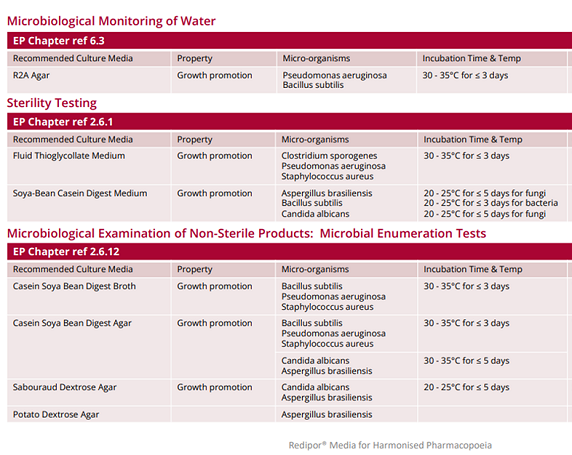
Harmonised Pharmacopoeia Culture Media
The European Pharmacopoeia (Ph. Eur.) is a reference work for the quality control of medicines. The official standards within it provide a scientific basis for quality control during the entire life cycle of a product. The general microbiology text in the Ph. Eur. is harmonised with the corresponding chapters of the United States and Japanese Pharmacopoeias, to enable the free movement and trade of medicines within Europe and other countries.
We have created a table to show the Redipor range of prepared media from Cherwell that is formulated and performance tested in accordance with the requirements stipulated within the European Pharmacopoeia. For example R2A agar for the microbiological monitoring of water; Soya-bean Casein Digest Medium (Tryptic Soy Broth) used in sterility testing; Sabouraud Dextrose Agar (SDA) and Casein Soya Bean Digest Agar (TSA) for microbial enumeration tests and culture media to test for specified micro-organisms, such as MacConkey agar and broth used for E. coli.
R2A
This low nutrient medium is designed for the recovery of organisms from water which may have been stressed by water treatment solutions. Proven to be more effective than more nutritious media, this agar is typically incubated at lower temperatures for longer periods to help with recovery of those damaged organisms.
Developed by Reasoner and Geldreich, this pale coloured medium is used to assess the quality of processed water within pharma.
Relevant products:
Relevant content:
How Cherwell ensures consistent quality of all Prepared Media
How to Store Prepared Media Plates 

Failure is not an option: Why sterility testing is so important
In this eBook we look at why sterility testing is such an important requirement and address the role of your environmental monitoring program and how it is critical to the success of your sterility testing, product quality and reputation
FREQUENTLY
Asked Questions
Yes. We have almost thirty years' experience with helping pharmaceutical and associated industries deliver environmental monitoring processes critical to the manufacture of heavily regulated products.
We supply every component you need to create environmental monitoring processes. We also offer bespoke formulations, accessories and even packaging solutions for organisations with very particular needs.
Get in touch today. Let's discuss your requirements.
The majority of our prepared media can be stored in ambient conditions, not exceeding 25ºC. There are only a small number of very specialist products that require different storage conditions.
We have never specified refrigeration as a storage condition for our general media as this causes excess condensation and can result in very wet agar, rendering it impossible to use.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
General purpose media have nutrients that support the growth of most non fastidious culturable microorganisms.
Selective growth media contain components that will inhibit the growth of some types of microorganisms, while supporting the growth of others.
General purpose media, such as Tryptone Soya Agar, are used to produce total counts. While selective media, such as XLD for Salmonella species, are used to test presence/absence of specific types of microorganism.
Typical requirements suggest 1,000 litres per air sample in high risk areas, such as: grade A filling lines, grade B clean rooms, operating theatres etc. As the criticality of the area reduces, the sample size can be reduced. The aim is to achieve a representative sample; so where higher counts would be expected, a smaller sample produces a more realistic number of cfu to count.
SAS samplers were originally designed for Contact plates, however, a Petri dish option has been available for a number of years. It is really a personal choice, although this should be decided at time of purchase, as the sampler will be specifically configured for the plate type chosen. There are advantages for each version and we would be happy to discuss your specific needs.